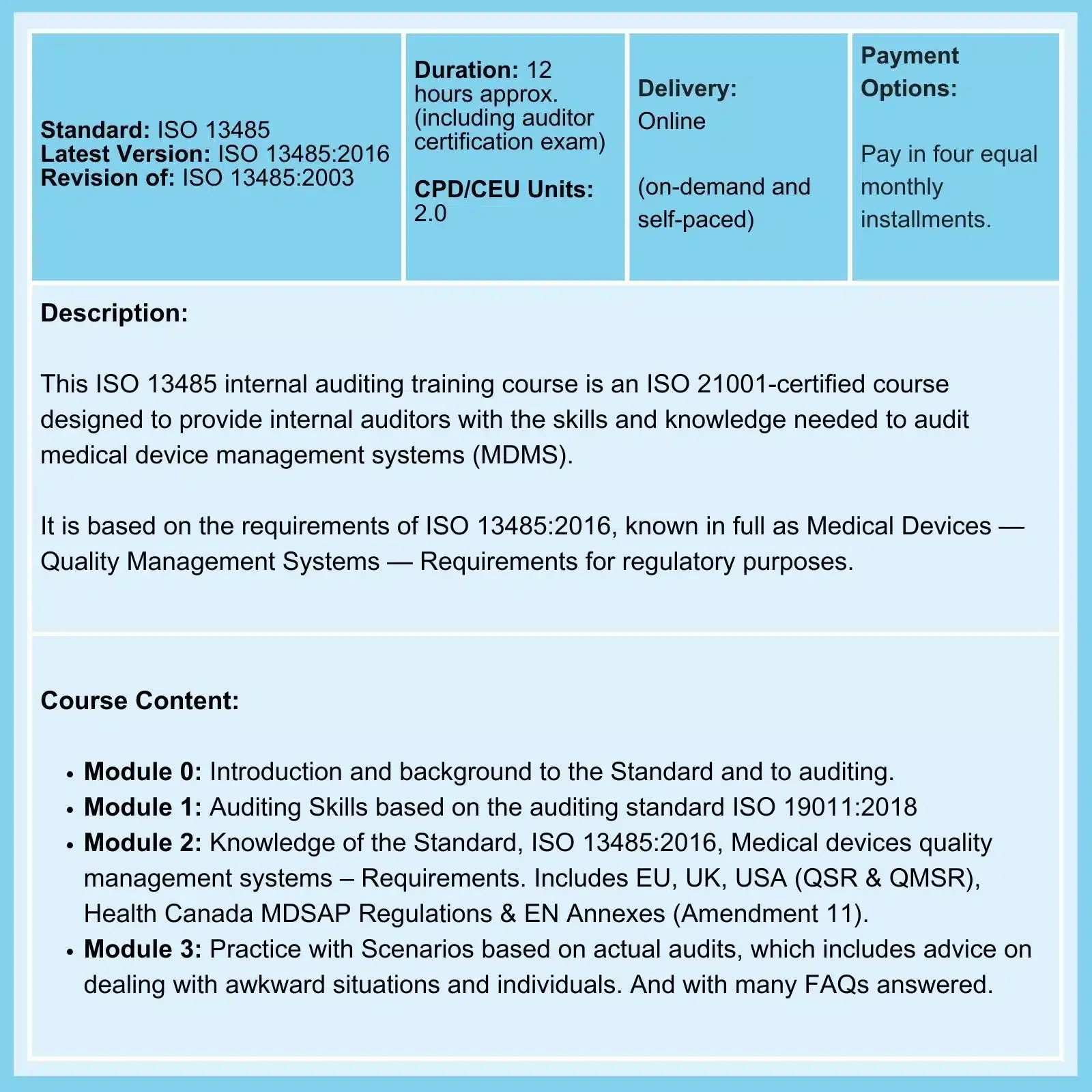
ISO 13485 2016 Lead Auditor Extension Course
With this Course, you can add ISO 13485 Lead Auditor certification to your current Lead Auditor Certification.
This will enable you to participate in Certification Body and Registration Body auditing. Already a Lead Auditor for ISO 9001 or other Management System? Add ISO 13485:2016 Lead Auditor Certification with this online program. It provides you with the Auditing Skills, the Knowledge of the Standard, Knowledge of Risk Management and associated Tools, and the practical application of that knowledge with Audit Scenarios to enable you, as Audit Team Leader, to undertake and manage Certification Audits.
Key Features:
- Course is accredited to ISO 21001, the standard for Educational Organizations Management Systems
- An ISO 13485 Lead Auditor certification is awarded upon passing the certification exam
- Certificate is immediately available online after the successful passing of the exam
- Certificate comes with a shareable QR code for instant verification of credentials
- Lessons range from 15 minutes to 1 hour, typically 20–30 minutes, ensuring that each topic is covered in suitable detail
- Course includes practice with scenarios that include dialogues
- Course includes 24/7 Live-chat Learner Support
- Course includes a learner manual, a copy of the standard, and samples of relevant forms and other documents
- Course comes with full-audio narration and Closed Captions for accessibility
- Courses are hosted on your browser so that no software has to be downloaded avoiding security risks.
- Course comes with quizzes, practice with scenarios, and open-book certification exam
- Course is hosted on your browser so that no software has to be downloaded avoiding security risks.
- Features cross-device compatibility (courses can be taken on any desktop, tablet, or mobile)
- Offers full-resume feature (end a session mid-lesson and continue exactly where you left off, even from a different device)
- Features real-time interactive content in a secure web-based environment
- Offers a clear learning path (once you've completed the internal auditor course, you have the option to progress to the lead auditor, up to the consultant and lead auditor course.
- Examination and certificate fee are already included in the course fee
- Payable via PayPal or Stripe using any credit or debit cards
- Option to pay in 4 monthly installments available
Who should enroll in this ISO 13485 Lead Auditor Extension Course?
This ISO 13485 Lead Auditor Extension course is intended for:- Senior quality managers
- Quality professionals
- Regulatory professionals
- Compliance professionals
- Project managers
- Design engineers
- Software engineers
- Process owners
- Quality engineers
- Quality auditors
- Medical affairs
- Legal Professionals
What will I learn from this ISO 13485 Lead Auditor Extension Course?
This comprehensive ISO 13485 Lead Auditor Extension course is divided into four modules:- Module 0: Introduction and background to the Standard and to Auditing.
- Module 1: Knowledge of the Standard, ISO 13485:2016, Medical Devices – Quality management systems – Requirements for regulatory purposes:
- Introduction to ISO 13485:2016 (Includes EU, UK, USA Regulations & MDSAP)
- Developing & Implementing a QMS
- Terms & Definitions – ISO 13485:2016
- Some key aspects of QMS Auditing
- Fundamentals of Medical Device Management Systems
- Structure & content of ISO 13485:2016
- Parts 1, 2 & 3
- Part 4: Quality Management System Requirements
- Part 5: Management Responsibility
- Part 6: Resources
- Part 7: Product Realisation (3 lessons)
- Part 8: Measurement, Analysis & Improvement (2 lessons)
- Annexes ZA & ZB (New Anneses Amendment 11)
- Advanced aspects of QMS Auditing (3 lessons)
- FAQs about the Standard
- Online Course Examination
- Module 2: Practice with Scenarios, based on actual audits and includes advice on dealing with awkward situations and individuals.
- Audit Scenarios – Internal Auditor
- More Audit Scenarios – Lead Implementers
- Yet more Audit Scenarios – Lead Auditors
- FAQs about the Auditing Experience
- Online Final Examination
What materials are included in this ISO 13485 Lead Auditor Extension Course?
This ISO 13485 Lead Auditor Extension course comes with:- Diagram: ISO Auditor Certification Process
- Diagram: 6-Stage Audit Process
- Sample Code of Ethics
- Management of an Audit Programme
- Sample Audit Plan
- Sample Audit Work Order
- Sample Nonconformity Report
- Sample Working Document & Checklist
- Risk Assessment Tools and Methodologies
- Documented Information Requirements of ISO 13485:2016
- Determining the Context of the Organization
- ISO 13485:2016 and Risk Management
- Risk Assessment Tools and Methodologies (with examples)
- Terms & Definitions
- Typical Process Map
How is this ISO 13485 Lead Auditor Extension Course delivered?
This ISO 13485 Lead Auditor Extension course is delivered online from our Learning Management System (LMS) at www.degrandsonLMS.com (provided by Inquisiq). All Lessons have a full resume and scaling capabilities. This means, for example, you can:- Start a Lesson at work on your Work Station running on Windows 10,
- Continue the Lesson on the train home on your iPad running on iOS 11 and,
- Complete the Lesson at home on your Notebook PC running on Windows 8.1.
Are there any prequalifications to enroll in this ISO 13485 Lead Auditor Extension Course?
If you have previously completed a Lead Auditor Program with deGRANDSON Global, you are pre-qualified for this ISO 13485 lead auditor extension program. Just log-in, enroll and start your program immediately.
If you have NOT previously completed an Internal Auditor Program with deGRANDSON Global, you are advised to complete a free Pre-Test first on order to demonstrate adequate auditing skills.
The Pre-Test will take 30 minutes approx. and is FREE. Click this link to begin: Check your Lead Auditor Skills.